GRETNA – A man is suing two pharmaceutical companies over side effects he claims to have suffered due to taking an anti-depressant.
Cory Jenkins filed suit against Bristol-Myers Squibb Company and Outska America Pharmaceutical Inc. in the 24th Judicial District Court on Oct. 17.
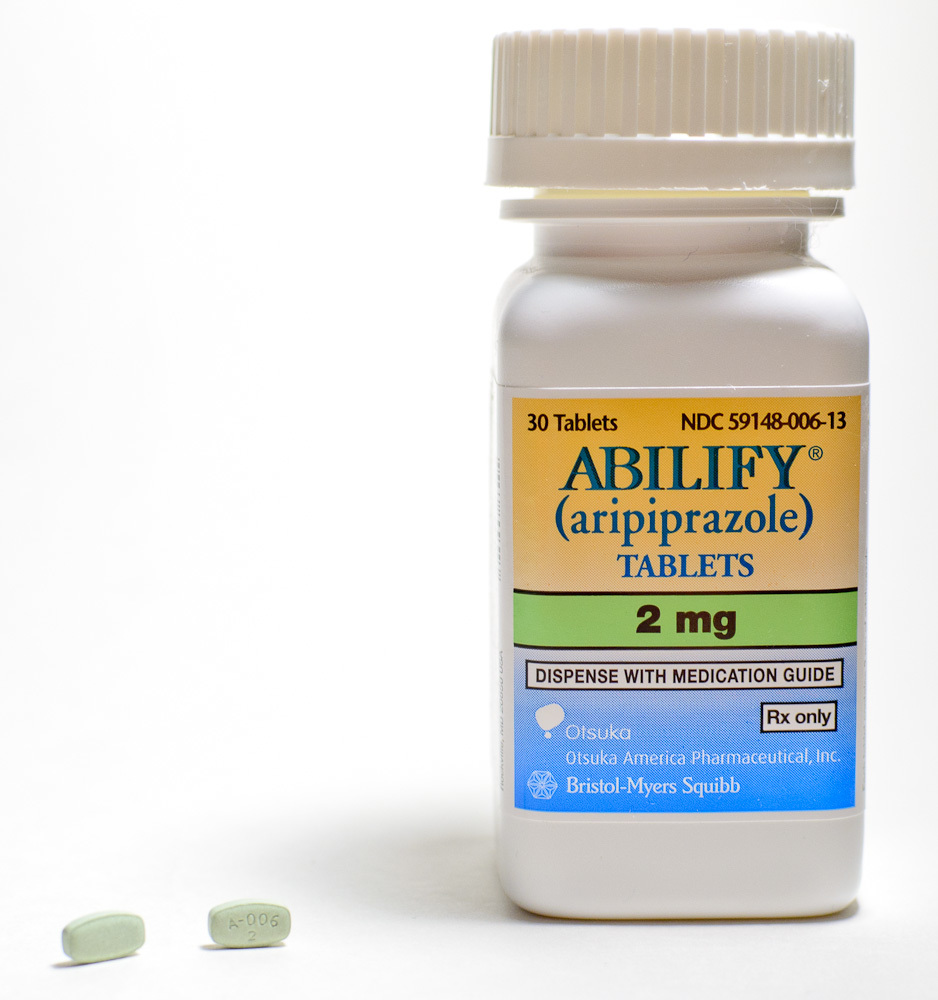
Jenkins contends that he was prescribed a two milligram dose per day of Abilify on Oct. 19, 2010 to treat his bipolar disorder. The plaintiff asserts that in October 2013 he began to develop symptoms of restlessness and twitching in his extremities that progressed and was eventually diagnosed as Tardive dyskinesia, an incurable movement disorder thought to be brought on by long-term use of antipsychotic drugs. Jenkins claims that in early 2014 he was told that the condition was caused by his taking Abilify.
The defendant is accused of creating and selling a drug unreasonably dangerous in design, inadequate warning and failure to warn of Tardive dyskinesia and inadequate warnings as to the procedures necessary to monitor patients while taking Abilify.
An unspecified amount in damages is sought for medical expenses, lost wages and general damages.
Jenkins is represented by Perry R. Staub Jr. of New Orleans-based Taggart Morton LLC.
The case has been assigned to Division O Judge Ross P. LaDart.
Case no. 743-490.
Man files lawsuit claiming antidepressant Abilify caused neurological disorder
ORGANIZATIONS IN THIS STORY